ROB THOBURN | | | |
| |
| |
Myostatin. Heard of it?
Whether you have or not, myostatin is circulating inside your body at
this very moment…and it’s got a growth-restraining order on your muscles.
The tiny myostatin protein conveys a powerful biological message to
your muscle cells -and it’s not anything along the lines of “let’s get
ready to grow”.
Quite the contrary, myostatin is proving to play a major role in your
body’s resistance to bigger muscles, i.e., your failure to grow
to the dimensions you truly desire. However, as this article will show,
recent technological developments may make it possible for you to enjoy
‘hypertrophic freedom’ in the not-too-distant future. Planet Muscle,
of course, will be the first to report on these industry-shaking developments.
Did you feel that? Another myostatin molecule just fit itself neatly
into a receptor on the membrane of your muscle cell. Oops! There goes another
one! Right now, many thousands of such myostatin-receptor interactions
are triggering a flurry of biochemical events that will serve to keep your
muscle cells from getting as big as you’d like.
“But I work out like a maniac! I eat 300 grams of protein a day!”, you
protest. Which is all fine and good. Yet your gains in muscle size are
unsatisfactory, are they not? Yes, and myostatin has a great deal to do
with this.
choke collar
n.
A chain collar that tightens like a noose when the leash is pulled.
Used to train or control dogs or other animals. Also called choke chain.
myostatin
n.
A protein, the product of the myostatin gene, that tightens like a
noose on the protein-building machinery of muscle cells. Used to limit
and restrict muscle growth in adults. Generally detested by bodybuilders.
Also called the skinny protein.
Indeed, myostatin could well be regarded as Mother Nature’s ‘choke collar’
on muscle growth (Whittmore et al., 2003). Hence, the ‘skinny protein’.
Myostatin powerfully suppresses the protein-building machinery of your
muscle cells (more on this later), restricting them to only a very limited
degree of growth. In this way, myostatin effectively keeps your muscles
from getting bigger than is necessary to cope with the average situation
reasonably well.
Okay, maybe not good enough for you and I, but certainly good enough
for Mother Nature.
Yes, biological evolution -natural selection, if you like- favors not
the magnificent, but the moderate. The human body got us to this
point because it could cope with the average situation faced by
our ancestors tens to hundreds of thousands of years ago reasonably
well. This reasonable coping power is programmed into the supercoiled
helices of your very own DNA - your genes. Genes, furthermore, which carry
much the same messages that they carried in your prehistoric kin.
The trouble is that socially and culturally, things are
anything but the same. Your modern-day wants frequently clash with what
your biological needs were for the bulk of your evolutionary journey. In
short, human biology hasn’t caught up with the times! Thus, bodybuilders
like you and I have desires for muscular size (among others) that often
far exceed what our genes are able to pony up.
Bodybuilding is about anything
but moderation, or coping ‘reasonably
well’. It is
all about grandiosity, both in spirit (ego) and in
physical dimensions. Just visit the lobby of the Mandelay Bay hotel on
Mr. Olympia Weekend to see what I mean!
While myostatin may be helpless at keeping your ego on a leash, it does
an exceptional job at negatively modulating, or restricting, the growth
of your muscles. Only with years of exhaustive training and entire chicken
farms of protein do your hair-like muscle cells show substantial increases
in diameter. Even then, the gains can be annoyingly small --perhaps even
unnoticeable to you and your all-too-critical peers.
Every day I pass by tiny shrubs, bushes and other caricatures of plant
life as I walk down the street. I look at them and wonder: Do any of these
plants harbor a myostatin ‘neutralizer’? Is nature hiding a substance with
which to effectively break free of the choke collar on my muscle-building
machinery?
Maybe. Maybe not. In any case, the search for a myostatin ‘neutralizer’
is certainly worthwhile. It is also well underway (see below).
Case in point: Studies on animals harboring mutations in the myostatin
gene. These animals demonstrate dramatically enhanced muscle growth and
an equally astonishing reduction in body fat -precisely what you and I
are after.
Arnold et al. (2001) refer to myostatin ‘knockout’ mice (i.e., mice
with a deleted myostatin gene) that display
“bulging muscular development visible all over their bodies,
with the most extreme hypertrophy apparent in the shoulders and hindquarters…”
They go on to describe the so-called ‘double-muscled cattle’ that “are
even more easily discernable than their murine [mouse] counterparts. On
Belgian Blue bulls,
every intramuscular groove is readily visible, due to an
almost complete lack of subcutaneous fat. Instead of the "boxy" build of
typical cattle, double-muscled animals have tight, "greyhound" bellies…Indeed,
they have a muscular conformation most often reserved for draft horses
and bodybuilders - and such a blatant advertisement of retail product that
the industry interest is obvious.”
Dr. Arnold’s group adds that the dramatic muscle growth seen in myostatin
‘null’ animals is specific to muscle tissue. Furthermore, the hypertrophy
is observed throughout the body, and not merely specific to certain regions.
That’s an important question. Like the muscle tissue in some patients
with growth-hormone secreting tumors, will the muscle created by myostatin
inhibition be larger than normal, but weaker than expected?
Bogdanovich et al. (2002) provide some evidence to suggest that myostatin
blockade will result in muscles that are as big as they are strong. When
they injected mice with muscular dystrophy with an antibody to myostatin
for three months, they found that:
“[the] increase in muscle strength was proportional to the
degree of increase in muscle mass and offers physiological evidence for
a functional improvement in [muscular dystrophic] muscle produced by myostatin
blockade in vivo.”
NOTE: For additional evidence with which to support the notion that
myostatin blockade produces muscles that are as big as they are strong,
see Whittmore et al. (2003) under REFERENCES.
Being a regulator of the growth and division of your muscle cells, you
might expect the risk of side effects (e.g., cancer) upon ‘successfully’
blocking myostatin from doing its job.
Myostatin belongs to the transforming growth factor (TGF) family of
growth factors. Like myostastin, transforming growth factor-beta (TGF-b)
can act as a ‘choke collar’ on cell growth.
As one well-known (and technically articulate) industry expert remarked
to me “Many substances have impact on TGF-b pathways but are hazardous
as they can promote growth and survival of dysplastic and frankly malignant
clonal populations.”
In other words, if you block myostatin, you may produce runaway cell
division, also known as cancer.
The evidence to date, however, suggests that myostatin blockade will
not produce cancer. Dr. Clifton Baile (2003), a Distinguished Professor
of Animal Science and Foods and Nutrition at the University of Georgia,
comments:
“Myostatin is also referred to as GDF-8, a subclass of at
least 16 growth factors of the TGF gene family. The family of proteins
as a composite has a plethora of actions. Some have activities [of
those noted above] but not by GDF-8 or as the result of the inhibition
of GDF-8.
There have been no increased incidence of tumors of any kind reported
for any of the various types of mice produced where GDF-8 is inhibited
or in any cattle breeds where no or mutated and inactive GDF-8 is produced.
Specificity of the action of an inhibitor for GDF-8 may be important and
one of the most difficult qualities to obtain.”
When your body makes, or synthesizes, more muscle protein than it breaks
down over time, we generally expect bigger muscles to result.
There’s more to it than that. You may have already heard of satellite
cells. For our purposes, you can think of them as ‘baby’, or immature,
muscle cells. Satellite cells can divide to make copies of themselves.
This is termed proliferation. They can also merge with your ‘adult’,
or mature, muscle cells and donate their DNA in times of need.
In fact, the proliferation of your satellite cells is essential for
muscle growth (Allen et al., 1979). [NOTE: Since satellite cell
proliferation increases the total amount of DNA in a muscle (Oksbjerg et
al., 2002), and the protein synthesis capacity is reflected in the RNA
levels (Millward et al., 1973), we can measure these two things to get
a feel for the anabolic state of a muscle cell.]
Ah, yes, the ones with algae in them.
Perhaps you’ve heard of glucosamine? The stuff for your joints?
Heparin is a carbohydrate (a.k.a. ‘polysaccharide’) that occurs all
over your body, especially in the lining of your blood vessels and the
spaces between your cells. (Many scientists believe that heparin is not
active when taken orally. But in fact, Canadian researchers have determined
that this isn’t entirely true.)
One of heparin’s building blocks is glucosamine. Heparin often occurs
with sulfur attached to it, i.e., heparin sulfate. Thus, heparin sulfate
may be referred to as a ‘sulfated polysaccharide’.
Many different growth-regulating proteins in your body, including myostatin,
can bind to heparin. These heparin interactions may serve to ‘store’ the
proteins until they are needed, concentrate them near their receptors (e.g.,
IGF-1 near the IGF-1 receptor) and/or protect them from inactivation by
protein-digesting enzymes.
The sites on the protein that make heparin binding possible are called,
appropriately enough, ‘heparin binding sites’. Heparin binding sites are
very common in growth-regulating proteins, and have been used to purify
myostatin.
So-called myostatin ‘inhibitors’ currently on the market contain a ‘sulfated
polysaccharide’, analogous to heparin sulfate, from a ‘natural’, ‘exotic’
salt-water algae. The ads claim that this sulfated polysaccharide binds
myostatin better than heparin. They go on to say that because the interest
in this product was so extensive, they decided to dispense with any further
studies and let the public buy this miraculous product immediately.
You be the judge. Does that sound like direct clinical proof of efficacy
to you?
Of course, many substances in nature (possibly even inside your fridge)
may well show strong myostatin binding activity. But until we do a double-blind
clinical trial on humans and demonstrate the oral activity of such a substance
---most importantly, its ability to increase muscle mass and reduce fat
mass-we’re just theorizing, at best.
One fact overlooked by marketers of existing ‘myostatin inhibitors’
is that myostatin seems to circulate in the blood as a latent, or inactive,
complex (Whittmore et al., 2003). Even if you consumed enough of a ‘sulfated
polysaccharide’ to transiently bind a biologically significant amount of
myostatin, what would that do to its activity when it is already in a latent
complex? I don’t know. Do you?
Questions remain, to be sure. And products that tout claims like “we
were too bloody excited about this stuff to do any studies on it” do nothing
but stall the answering of these questions.
Myostatin seems to put a choke collar on the activity of your satellite
cells and the protein-building machinery of your mature muscle cells. Release
this choke collar, therefore, and your anabolic devices will presumably
be free to engage in muscle growth at a pace Mother Nature never intended.
“To date…myostatin is the only secreted protein that has
been demonstrated to play a negative role in regulating muscle mass in
vivo. Although additional experiments will be required to prove aspects
of this overall model and to identify the other signaling components, our
data suggest that myostatin antagonists, such as follistatin and the myostatin
propeptide, or activin type II receptor antagonists may be effective muscle-enhancing
agents for both human and agricultural applications.” (Lee and McPherron,
2001).
To use the phraseology of Drs. Lee and McPherron, ‘antagonizing’ myostatin
is precisely what one University-based company is planning on doing.
This group of anabolically-oriented scientists (let’s call them ‘Newco’)
is developing a number of in vitro (cell-based) assays with which to screen
for natural, orally bioavailable products with myostatin-‘neutralizing’
activity. In vivo (whole animal) assays -ultimately involving human beings-will
be developed to confirm the findings of the in vitro work.
In other words, and uniquely, the product(s) discovered by Newco will
hit the shelf neatly dressed in direct clinical proof of efficacy ---proven
to do something of compelling important to you (i.e., building muscle,
removing fat).
|
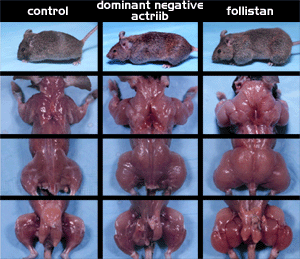
Figure 1. A muscle ‘explosion’ follows myostatin ‘neutralization’.
The control mouse (normal) is shown for comparison. The ActRIIB mice have
a genetic defect that prevents myostatin from binding to its purported
receptor. Follistatin mice are genetically modified to express high levels
of follistatin; this also results failure of myostatin to bind to its receptor.
The result in both cases is dramatically enhanced muscle mass. Similar
studies indicate large reductions in body fat. Strength and caloric output
also increase markedly.
From: Lee S-J, McPherron AC (2001). Regulation of myostatin
activity and muscle growth. Proc Natl Acad Sci, USA 98: 9306. Image used
with permission from the Proceedings of the National Academy of Sciences,
USA. Copyright 2003. National Academy of Sciences, U.S.A. |
-
Increases in muscle mass with proportional increases in muscle strength
(big muscles that are as strong as they look)
-
Reductions in body fat (get much leaner)
-
Increases in caloric output (eat more without getting fat)
All too frequently, bodybuilding, weight-loss and other dietary supplements
come to you, the consumer, naked of any direct scientific support. This
not only cheats you; it also cheats and undermines the industry as a whole.
To repeat, any products touting claims for myostatin ‘neutralization’,
‘inhibition’, or the like must be accompanied by direct clinical proof
of efficacy. This means confirmation of myostatin inhibition by in
vitro work, followed by confirmation of safety and body composition enhancement
in animal models and, ultimately, human clinical trials.
Furthermore, we want specific inhibition of myostatin. We don’t
want to ‘neutralize’ any other cell proliferation/differentiation regulating
substances. That could lead to illness. As Dr. Baile explains, specific
inhibition of myostatin may be the most difficult thing to screen for.
But it’s possible.
The potential applications of Newco’s technologies are tremendous: obesity,
type II diabetes, muscular dystrophies and other conditions of muscle wasting
(e.g., cancer, AIDs, space travel), and of course, bodybuilding and general
weight-management.
Will there soon be safe, orally active, natural products capable of
inhibiting myostatin and freeing your muscles to grow as you desire?
Don’t miss an issue of Planet Muscle, and you’ll be assured
of finding out!
REFERENCES
Arnold HA, Della-Ferra MA, Baile C (2001). Review of myostatin history,
physiology and applications. LifeXY, 2001(1): 1014. available at: http://www.lifexy.com/aa/P10619-11065.pdf.
Baile C (2003). Personal communication.
Bodgdanovich S, Krag TOB, Barton ER et al. (2003). Functional improvement
of dystrophic muscle by myostatin blockade. Nature, 420: 818.
Lee S-J, McPherron AC (2001). Regulation of myostatin activity and
muscle growth. Proc Natl Acad Sci, USA 98: 9306.
Whittemore LA, Song K, Li X et al. (2003). Inhibition of myostatin
in adult mice increases skeletal muscle mass and strength. Biochem Biophys
Res Commun, 300: 965.
© 2003 Rob Thoburn. All Rights Reserved.